| CAT No | Product | Size | Price |
|---|---|---|---|
| PB25.41-01 | qPCRBIO Probe 1-Step Go Lo-ROX | 100 x 20 μL Reactions | Contact us |
| PB25.41-03 | qPCRBIO Probe 1-Step Go Lo-ROX | 300 x 20 μL Reactions | Contact us |
| PB25.41-05 | qPCRBIO Probe 1-Step Go Lo-ROX | 500 x 20 μL Reactions (1 x 5 mL) | Contact us |
| PB25.41-12 | qPCRBIO Probe 1-Step Go Lo-ROX | 1200 x 20 μL Reactions | Contact us |
| PB25.41-50 | qPCRBIO Probe 1-Step Go Lo-ROX | 5000 x 20 μL Reactions (1 x 50 mL) | Contact us |
| PB25.42-01 | qPCRBIO Probe 1-Step Go Hi-ROX | 100 x 20 μL Reactions | Contact us |
| PB25.42-03 | qPCRBIO Probe 1-Step Go Hi-ROX | 300 x 20 μL Reactions | Contact us |
| PB25.42-05 | qPCRBIO Probe 1-Step Go Hi-ROX | 500 x 20 μL Reactions (1 x 5 mL) | Contact us |
| PB25.42-12 | qPCRBIO Probe 1-Step Go Hi-ROX | 1200 x 20 μL Reactions | Contact us |
| PB25.42-50 | qPCRBIO Probe 1-Step Go Hi-ROX | 5000 x 20 μL Reactions (1 x 50 mL) | Contact us |
| PB25.43-01 | qPCRBIO Probe 1-Step Go No-ROX | 100 x 20 μL Reactions | Contact us |
| PB25.43-03 | qPCRBIO Probe 1-Step Go No-ROX | 300 x 20 μL Reactions | Contact us |
| PB25.43-05 | qPCRBIO Probe 1-Step Go No-ROX | 500 x 20 μL Reactions (1 x 5 mL) | Contact us |
| PB25.43-12 | qPCRBIO Probe 1-Step Go No-ROX | 1200 x 20 μL Reactions | Contact us |
| PB25.43-50 | qPCRBIO Probe 1-Step Go No-ROX | 5000 x 20 μL Reactions (1 x 50 mL) | Contact us |
| PB25.44-01 | qPCRBIO Probe 1-Step Go Separate-ROX | 100 x 20 μL Reactions | Contact us |
| PB25.44-03 | qPCRBIO Probe 1-Step Go Separate-ROX | 300 x 20 μL Reactions | Contact us |
| PB25.44-05 | qPCRBIO Probe 1-Step Go Separate-ROX | 500 x 20 μL Reactions (1 x 5 mL) | Contact us |
| PB25.44-12 | qPCRBIO Probe 1-Step Go Separate-ROX | 1200 x 20 μL Reactions | Contact us |
To view your price, please login or register with your quote reference.
Additional Information
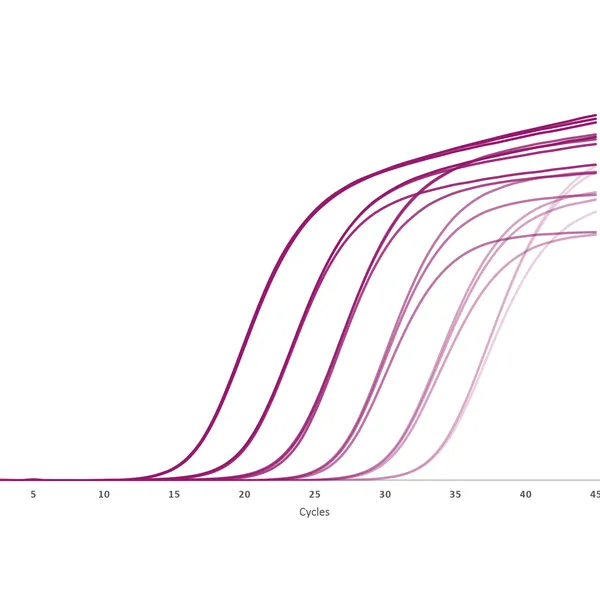
qPCRBIO Probe 1-Step Go is a versatile probe kit designed for rapid, highly specific and sensitive probe-based real-time RT-PCR. We use the latest advancements in reverse transcriptase technology and buffer chemistry to provide efficient cDNA synthesis and real-time PCR in a single tube.
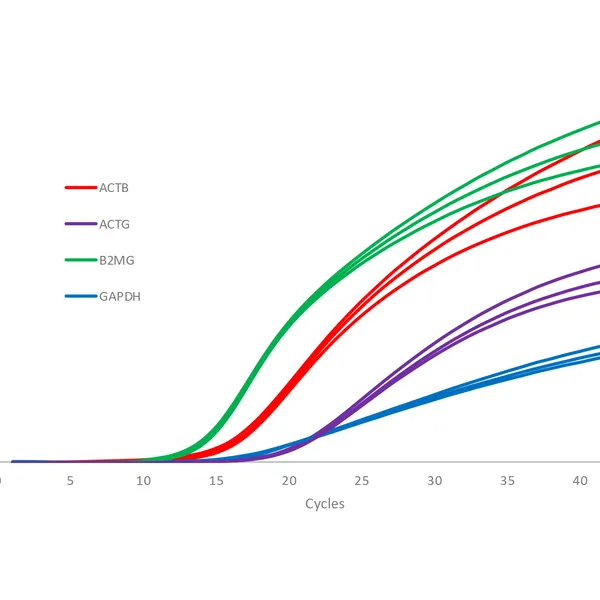
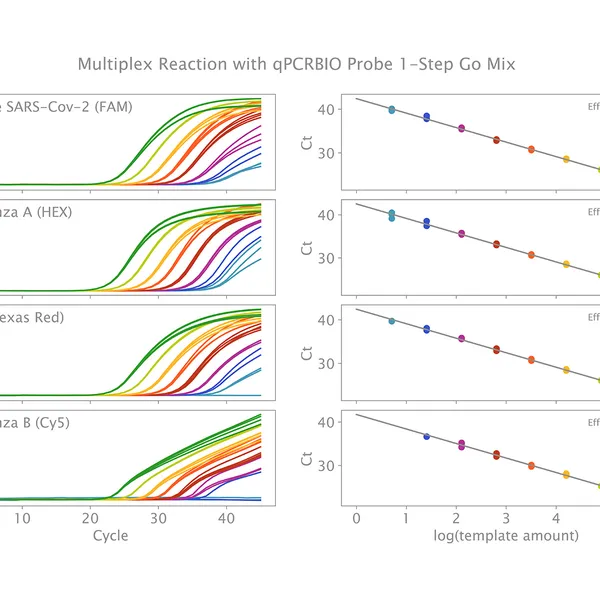
qPCRBIO Probe 1-Step Go is designed for use across a range of different probe technologies including TaqMan®, Scorpions®, and molecular beacon probes. The kit includes a 2x mix and can be used to quantify any RNA sample including mRNA, synthetic RNA, and viral RNA sequences. qPCRBIO Probe 1-Step Go is designed to provide fast and accurate results over a broad range of template concentrations and is particularly suited for the detection of RNA viruses including SARS-CoV-2.
The kit includes a modified MMLV reverse transcriptase with improved thermostability and ultra-high activity (RTase Go), along with an advanced RNase inhibitor to prevent RNA degradation by contaminating RNase. Antibody hot-start technology prevents the formation of primer-dimers and non-specific products, resulting in highly specific and ultra-sensitive real-time RT-PCR with unmatched performance in multiplex reactions. By combining the latest developments in polymerase technology and advanced buffer chemistry, we provide market-leading performance with little or no optimization required.
Applications
- COVID-19 / SARS-CoV-2 Testing and Research
- Diagnostical real-time RT-PCR
- Single or multiplex quantification
- Detection of low abundance targets
- Absolute quantification
- Relative gene expression analysis
- TaqMan®, Scorpions®, and molecular beacon probes
Technical Specifications
qPCRBIO Probe 1-Step Go Lo-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | 5000 Reactions | 50000 Reactions |
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go Lo-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | 1 x 50 mL | 1 x 500 mL |
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 3 x 100 μL | 1 x 500 μL | 12 x 100 μL | 1 x 5 mL | 1 x 50 mL |
qPCRBIO Probe 1-Step Go Hi-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | 5000 Reactions | 50000 Reactions |
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go Hi-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | 1 x 50 mL | 1 x 500 mL |
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 3 x 100 μL | 1 x 500 μL | 12 x 100 μL | 1 x 5 mL | 1 x 50 mL |
qPCRBIO Probe 1-Step Go No-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | 5000 Reactions | 50000 Reactions |
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go No-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | 1 x 50 mL | 1 x 500 mL |
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 3 x 100 μL | 1 x 500 μL | 12 x 100 μL | 1 x 5 mL | 1 x 50 mL |
qPCRBIO Probe 1-Step Go Separate-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | ||
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go No-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | ||
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 1 x 300 μL | 1 x 500 μL | 4 x 300 μL | ||
| 50μM ROX Additive | 1 x 200 μL | 1 x 200 μL | 1 x 200 μL | 4 x 200 μL |
Reaction Information
| Reaction Volume | Storage | |||
|---|---|---|---|---|
| 20μL |
Upon receipt, store product between -30 and -20 °C. When stored correctly, the kit will retain full activity until the expiration date indicated. |
Instrument Compatibility
This product is compatible with all standard and fast qPCR instruments. Use our qPCR Selection Tool to determine which ROX variant is compatible with your device.
Documents
Product Flyer
Manuals
Frequently Asked Questions (FAQs)
At what Ct value is the result considered unreliable?
Ct values can vary depending on the sample concentration, reaction optimization, instrument, and laboratory conditions, so caution should be taken when choosing a support cutoff Ct value. Generally, Ct values above 35 are considered unreliable. However, late Ct values may be observed for inefficient reactions using low sample amounts. It is always a good practice to standardize a cutoff with relative or absolute quantification methods. Running and analyzing melting curves or gels of the products to identify products from any late amplification is also recommended.
Can qPCRBIO Probe 1-Step Go be used for both one-step and two-step RT-PCR reactions?
No, this kit will not work for two-step reactions. For two-step reactions, we recommend using UltraScript® cDNA Synthesis Kit (PB30.11) or UltraScript® 2.0 cDNA Synthesis Kit (PB30.31) for more difficult targets, along with a real-time PCR kit depending on your needs.
Does ROX negatively impact the reaction?
ROX (6-carboxy-X-rhodamine) is used as a passive reference dye in real-time PCR instruments that rely on ROX to normalize for fluctuations in fluorescence that may arise mainly due to optical variation between wells. Fluorescence intensity normalization (Rn) is carried out in real-time PCR software by dividing the fluorescence emission of the specific signal by the fluorescence emission of ROX.
ROX does not participate in the PCR reaction and its fluorescence intensity is not proportional to the amount of DNA in each well, hence its addition to the mix provides a constant fluorescence signal throughout the amplification process.
Different real-time PCR instruments require a different concentration of passive reference standard of ROX, mainly due to different optical configurations of each system (i.e., different excitation and optical source types used).
Adding too little or too much ROX will result in a very noisy signal that affects reaction results. Therefore, it is very important that users:
- Determine the correct concentration of ROX to optimize real-time PCR results,
- Check the ROX setting in the software used to set up the reaction.
A useful selection tool for the most commonly used systems can be found here.
Can the activation time for HS Taq DNA polymerase be varied?
We recommend using at least 2 minutes for polymerase activation. Longer times up to 15 minutes can also be used with no negative effects on the enzyme.
Do I need a one-step or two-step reaction?
One-step
Both cDNA synthesis and PCR take place in the same mixture. This option is suitable for high-throughput applications due to the speed and simplicity of setup. There’s also a reduced risk of contamination. It is not ideal for low-quality RNA samples or if the cDNA is required for separate storage or analysis.
Two-step
cDNA synthesis and PCR occur separately. This option is preferable if cDNA product needs to be retained for analysis. It also allows for a greater degree of reaction optimization. Control over the type and concentration of enzyme, RNA input, and cDNA concentration allow for higher sensitivity compared to a one-step format.
Do I need to use an RNase inhibitor in my RT reaction?
No, RTase Go contains an RNase inhibitor to prevent any degradation and increase sensitivity.
General troubleshooting for low yield/late Ct values.
If you observe anomalously late Ct values, try diluting the sample RNA. By doing so, you are diluting any inhibitors that may be present to a level where they do not inhibit the reaction. Also, try increasing the reverse transcription step to 55 °C and increasing the annealing/extension temperature. This may help overcome difficulties caused by secondary structures present in RNA samples and/or primers.
In instances where reaction inhibition is suspected, try reducing sample volume1 or adding 0.4 – 4.4 mg/ml BSA to the reaction2.
For more specific queries, please email technical@pcrbio.com with the following information:
- Amplicon size
- Reaction setup
- Cycling conditions
- Screenshots of amplification traces and melting profiles
1 Scipioni et al. A SYBR Green RT-PCR assay in single tube to detect human and bovine noroviruses and control for inhibition. Virology Journal.5:94 (2008). doi: 1186/1743-422X-5-94
2 Plante et al. The use of bovine serum albumin to improve the RT‐qPCR detection of foodborne viruses rinsed from vegetable surfaces. Applied Microbiology. 52:3 (2010) doi: https://doi.org/10.1111/j.1472-765X.2010.02989.x
Is it normal for the fluorescence of qPCRBIO Probe Mixes to differ from competitor products?
Different products may yield different fluorescence plateaus. However, this does not affect quantification accuracy and Ct values will not vary between products.
Is it necessary to isolate mRNA for sensitive RT-PCR?
mRNA isolation is generally not necessary. The qPCRBIO Probe 1-Step Go kit has been developed to work on samples containing as little as 1 pg of total RNA or 0.01 pg of mRNA.
If you are working with rare mRNA species, consider using a gene-specific primer for the sequence in the RT reaction to increase sensitivity.
What is the difference between probe-based and dye-based mixes?
Probe-based kits like qPCRBIO Probe 1-Step Go offer higher sensitivity and are less likely to show non-template amplifications. Multiplexing can be measured using amplicons with different fluorophores for specific probes, which cannot be achieved with dyes.
Dye-based systems like qPCRBIO SyGreen 1-Step Detect | 1-Step Go detect any intact dsDNA and, therefore, will show primer-dimers and off-target/non-template amplifications. These can be separated from product peaks by melting curve analysis.
What is ROX and do I need it?
ROX is a passive reference dye which means it does not participate in the PCR reaction. It’s used to normalize non-PCR related fluctuations in fluorescence. You can use our qPCR Selection Tool from the Resources dropdown menu to determine which of our qPCR kits is right for your qPCR machine.
What is the concentration of MgCl2 in the qPCRBIO Probe 1-Step Go mixes?
All qPCRBIO Probe 1-Step Go mixes contain MgCl2 at a concentration of 9 mM. This means the final concentration in the reaction is 4.5 mM.
What type of primers can I use?
Gene-specific primers can be used in the one-step reaction.
What should I consider when performing multiplex amplification?
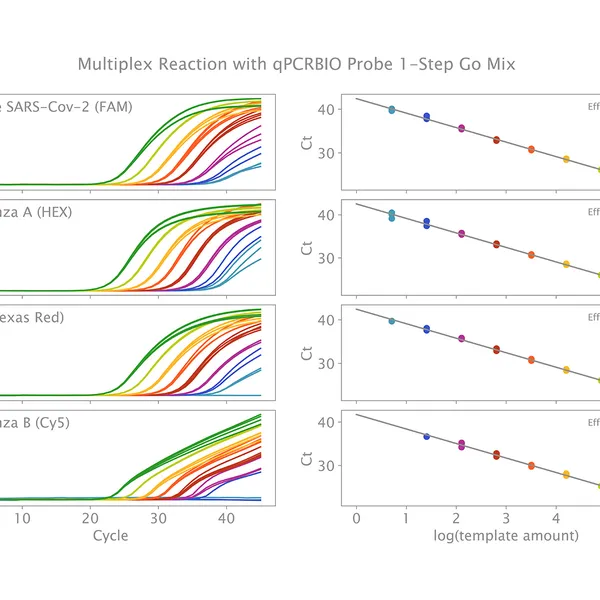
Primers should be designed to ensure they have similar annealing temperatures, are specific to the target, and do not form primer-dimers. Primer/probe concentrations should not require adjustment unless expression levels vary significantly. For targets with high expression levels, the primer amounts should be reduced and for lower levels, they should be increased.
What is the difference between PCRBIO 1-Step Go RT-PCR kit, qPCRBIO Probe 1-Step Go, and qPCRBIO SyGreen 1-Step Detect|Go?
The PCRBIO 1-step Go RT-PCR kit is developed for end-point RT-PCR. qPCRBIO Probe 1-Step Go and qPCRBIO SyGreen 1-Step Detect|Go are our probe-based and dye-based offerings for real-time RT-PCR, respectively.
When performing a multiplex, what is the recommended concentration of each primer?
We recommend using 0.4 µM for each primer. There is some flexibility around this recommended concentration; however, primer concentration should not be increased as this can significantly impact enzyme performance.
Does this work on micro RNA samples?
Yes, qPCRBIO Probe 1-Step Go can be used on micro RNA samples. Although we do not sell dedicated kits, all our RTases can be used for miRNA quantification and analysis.
We recommend one of two approaches:
- Using universal RT primers and adding a poly(A) or poly(U) tail (e.g., by poly(U) polymerase), followed by cDNA synthesis using universal primers1,2.
- Using specific RT primers and bypassing the tailing step1,3-5.
If you are unfamiliar with the details of these approaches, please see the list of references provided below, which will provide guidance.
1 Dave, V. P. et al. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Invest 99, 452-469, doi:10.1038/s41374-018-0143-3 (2019).
2 Mei, Q. et al. A facile and specific assay for quantifying microRNA by an optimized RT-qPCR approach. PLoS One 7, e46890, doi:10.1371/journal.pone.0046890 (2012).
3 Chen, C. et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33, e179, doi:10.1093/nar/gni178 (2005).
4 Raymond, C. K., Roberts, B. S., Garrett-Engele, P., Lim, L. P. & Johnson, J. M. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA 11, 1737-1744, doi:10.1261/rna.2148705 (2005).
5 Androvic, P., Valihrach, L., Elling, J., Sjoback, R. & Kubista, M. Two-tailed RT-qPCR: a novel method for highly accurate miRNA quantification. Nucleic Acids Res 45, e144, doi:10.1093/nar/gkx588 (2017).
Technical Specifications
qPCRBIO Probe 1-Step Go Lo-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | 5000 Reactions | 50000 Reactions |
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go Lo-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | 1 x 50 mL | 1 x 500 mL |
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 3 x 100 μL | 1 x 500 μL | 12 x 100 μL | 1 x 5 mL | 1 x 50 mL |
qPCRBIO Probe 1-Step Go Hi-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | 5000 Reactions | 50000 Reactions |
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go Hi-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | 1 x 50 mL | 1 x 500 mL |
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 3 x 100 μL | 1 x 500 μL | 12 x 100 μL | 1 x 5 mL | 1 x 50 mL |
qPCRBIO Probe 1-Step Go No-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | 5000 Reactions | 50000 Reactions |
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go No-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | 1 x 50 mL | 1 x 500 mL |
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 3 x 100 μL | 1 x 500 μL | 12 x 100 μL | 1 x 5 mL | 1 x 50 mL |
qPCRBIO Probe 1-Step Go Separate-ROX
| Component | 100 Reactions | 300 Reactions | 500 Reactions | 1200 Reactions | ||
|---|---|---|---|---|---|---|
| 2x qPCRBIO Probe 1-Step Go No-ROX | 1 x 1 mL | 3 x 1 mL | 1 x 5 mL | 12 x 1 mL | ||
| 20x RTase Go with RNase Inhibitor | 1 x 100 μL | 1 x 300 μL | 1 x 500 μL | 4 x 300 μL | ||
| 50μM ROX Additive | 1 x 200 μL | 1 x 200 μL | 1 x 200 μL | 4 x 200 μL |
Reaction Information
| Reaction Volume | Storage | |||
|---|---|---|---|---|
| 20μL |
Upon receipt, store product between -30 and -20 °C. When stored correctly, the kit will retain full activity until the expiration date indicated. |
Instrument Compatibility
This product is compatible with all standard and fast qPCR instruments. Use our qPCR Selection Tool to determine which ROX variant is compatible with your device.
Documents
Product Flyer
Manuals
Frequently Asked Questions (FAQs)
At what Ct value is the result considered unreliable?
Ct values can vary depending on the sample concentration, reaction optimization, instrument, and laboratory conditions, so caution should be taken when choosing a support cutoff Ct value. Generally, Ct values above 35 are considered unreliable. However, late Ct values may be observed for inefficient reactions using low sample amounts. It is always a good practice to standardize a cutoff with relative or absolute quantification methods. Running and analyzing melting curves or gels of the products to identify products from any late amplification is also recommended.
Can qPCRBIO Probe 1-Step Go be used for both one-step and two-step RT-PCR reactions?
No, this kit will not work for two-step reactions. For two-step reactions, we recommend using UltraScript® cDNA Synthesis Kit (PB30.11) or UltraScript® 2.0 cDNA Synthesis Kit (PB30.31) for more difficult targets, along with a real-time PCR kit depending on your needs.
Does ROX negatively impact the reaction?
ROX (6-carboxy-X-rhodamine) is used as a passive reference dye in real-time PCR instruments that rely on ROX to normalize for fluctuations in fluorescence that may arise mainly due to optical variation between wells. Fluorescence intensity normalization (Rn) is carried out in real-time PCR software by dividing the fluorescence emission of the specific signal by the fluorescence emission of ROX.
ROX does not participate in the PCR reaction and its fluorescence intensity is not proportional to the amount of DNA in each well, hence its addition to the mix provides a constant fluorescence signal throughout the amplification process.
Different real-time PCR instruments require a different concentration of passive reference standard of ROX, mainly due to different optical configurations of each system (i.e., different excitation and optical source types used).
Adding too little or too much ROX will result in a very noisy signal that affects reaction results. Therefore, it is very important that users:
- Determine the correct concentration of ROX to optimize real-time PCR results,
- Check the ROX setting in the software used to set up the reaction.
A useful selection tool for the most commonly used systems can be found here.
Can the activation time for HS Taq DNA polymerase be varied?
We recommend using at least 2 minutes for polymerase activation. Longer times up to 15 minutes can also be used with no negative effects on the enzyme.
Do I need a one-step or two-step reaction?
One-step
Both cDNA synthesis and PCR take place in the same mixture. This option is suitable for high-throughput applications due to the speed and simplicity of setup. There’s also a reduced risk of contamination. It is not ideal for low-quality RNA samples or if the cDNA is required for separate storage or analysis.
Two-step
cDNA synthesis and PCR occur separately. This option is preferable if cDNA product needs to be retained for analysis. It also allows for a greater degree of reaction optimization. Control over the type and concentration of enzyme, RNA input, and cDNA concentration allow for higher sensitivity compared to a one-step format.
Do I need to use an RNase inhibitor in my RT reaction?
No, RTase Go contains an RNase inhibitor to prevent any degradation and increase sensitivity.
General troubleshooting for low yield/late Ct values.
If you observe anomalously late Ct values, try diluting the sample RNA. By doing so, you are diluting any inhibitors that may be present to a level where they do not inhibit the reaction. Also, try increasing the reverse transcription step to 55 °C and increasing the annealing/extension temperature. This may help overcome difficulties caused by secondary structures present in RNA samples and/or primers.
In instances where reaction inhibition is suspected, try reducing sample volume1 or adding 0.4 – 4.4 mg/ml BSA to the reaction2.
For more specific queries, please email technical@pcrbio.com with the following information:
- Amplicon size
- Reaction setup
- Cycling conditions
- Screenshots of amplification traces and melting profiles
1 Scipioni et al. A SYBR Green RT-PCR assay in single tube to detect human and bovine noroviruses and control for inhibition. Virology Journal.5:94 (2008). doi: 1186/1743-422X-5-94
2 Plante et al. The use of bovine serum albumin to improve the RT‐qPCR detection of foodborne viruses rinsed from vegetable surfaces. Applied Microbiology. 52:3 (2010) doi: https://doi.org/10.1111/j.1472-765X.2010.02989.x
Is it normal for the fluorescence of qPCRBIO Probe Mixes to differ from competitor products?
Different products may yield different fluorescence plateaus. However, this does not affect quantification accuracy and Ct values will not vary between products.
Is it necessary to isolate mRNA for sensitive RT-PCR?
mRNA isolation is generally not necessary. The qPCRBIO Probe 1-Step Go kit has been developed to work on samples containing as little as 1 pg of total RNA or 0.01 pg of mRNA.
If you are working with rare mRNA species, consider using a gene-specific primer for the sequence in the RT reaction to increase sensitivity.
What is the difference between probe-based and dye-based mixes?
Probe-based kits like qPCRBIO Probe 1-Step Go offer higher sensitivity and are less likely to show non-template amplifications. Multiplexing can be measured using amplicons with different fluorophores for specific probes, which cannot be achieved with dyes.
Dye-based systems like qPCRBIO SyGreen 1-Step Detect | 1-Step Go detect any intact dsDNA and, therefore, will show primer-dimers and off-target/non-template amplifications. These can be separated from product peaks by melting curve analysis.
What is ROX and do I need it?
ROX is a passive reference dye which means it does not participate in the PCR reaction. It’s used to normalize non-PCR related fluctuations in fluorescence. You can use our qPCR Selection Tool from the Resources dropdown menu to determine which of our qPCR kits is right for your qPCR machine.
What is the concentration of MgCl2 in the qPCRBIO Probe 1-Step Go mixes?
All qPCRBIO Probe 1-Step Go mixes contain MgCl2 at a concentration of 9 mM. This means the final concentration in the reaction is 4.5 mM.
What type of primers can I use?
Gene-specific primers can be used in the one-step reaction.
What should I consider when performing multiplex amplification?
Primers should be designed to ensure they have similar annealing temperatures, are specific to the target, and do not form primer-dimers. Primer/probe concentrations should not require adjustment unless expression levels vary significantly. For targets with high expression levels, the primer amounts should be reduced and for lower levels, they should be increased.
What is the difference between PCRBIO 1-Step Go RT-PCR kit, qPCRBIO Probe 1-Step Go, and qPCRBIO SyGreen 1-Step Detect|Go?
The PCRBIO 1-step Go RT-PCR kit is developed for end-point RT-PCR. qPCRBIO Probe 1-Step Go and qPCRBIO SyGreen 1-Step Detect|Go are our probe-based and dye-based offerings for real-time RT-PCR, respectively.
When performing a multiplex, what is the recommended concentration of each primer?
We recommend using 0.4 µM for each primer. There is some flexibility around this recommended concentration; however, primer concentration should not be increased as this can significantly impact enzyme performance.
Does this work on micro RNA samples?
Yes, qPCRBIO Probe 1-Step Go can be used on micro RNA samples. Although we do not sell dedicated kits, all our RTases can be used for miRNA quantification and analysis.
We recommend one of two approaches:
- Using universal RT primers and adding a poly(A) or poly(U) tail (e.g., by poly(U) polymerase), followed by cDNA synthesis using universal primers1,2.
- Using specific RT primers and bypassing the tailing step1,3-5.
If you are unfamiliar with the details of these approaches, please see the list of references provided below, which will provide guidance.
1 Dave, V. P. et al. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Invest 99, 452-469, doi:10.1038/s41374-018-0143-3 (2019).
2 Mei, Q. et al. A facile and specific assay for quantifying microRNA by an optimized RT-qPCR approach. PLoS One 7, e46890, doi:10.1371/journal.pone.0046890 (2012).
3 Chen, C. et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res 33, e179, doi:10.1093/nar/gni178 (2005).
4 Raymond, C. K., Roberts, B. S., Garrett-Engele, P., Lim, L. P. & Johnson, J. M. Simple, quantitative primer-extension PCR assay for direct monitoring of microRNAs and short-interfering RNAs. RNA 11, 1737-1744, doi:10.1261/rna.2148705 (2005).
5 Androvic, P., Valihrach, L., Elling, J., Sjoback, R. & Kubista, M. Two-tailed RT-qPCR: a novel method for highly accurate miRNA quantification. Nucleic Acids Res 45, e144, doi:10.1093/nar/gkx588 (2017).


See more