| CAT No. | Product | Size | Price |
|---|---|---|---|
| PB90.02-03 | Lyo-Ready Probe Mix | 600 x 20 μL Reactions | Contact us |
| PB90.02-10 | Lyo-Ready Probe Mix | 2000 x 20 μL Reactions (2 x 5 mL) | Contact us |
| PB90.02-50 | Lyo-Ready Probe Mix | 10 000 x 20 μL Reactions (1 x 50 mL) | Contact us |
| PB90.13-03 | Pre-Lyo Probe 1-Step Evaluation Kit | 600 x 20 μL Reactions | Contact us |
| PB90.14-10 | Lyo-Ready Probe 1-Step Kit | 2000 x 20 μL Reactions (2 x 5 mL) | Contact us |
| PB90.14-50 | Lyo-Ready Probe 1-Step Kit | 10 000 x 20 μL Reactions (1 x 50 mL) | Contact us |
To see your price, please login or register with your quote code.
More Information
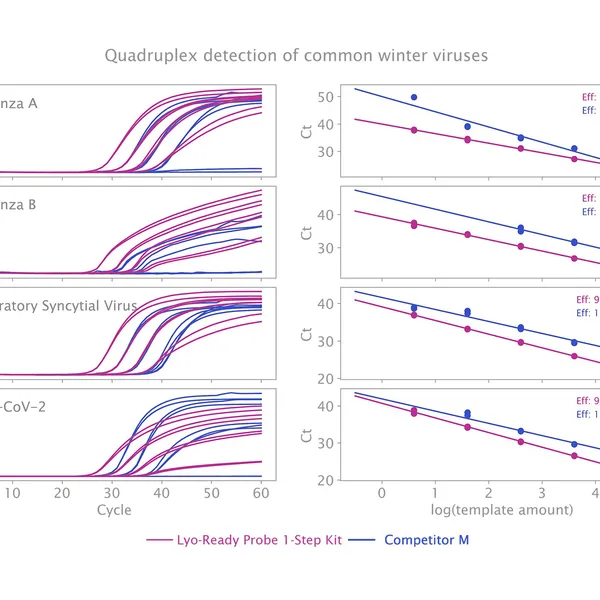
The Lyo-Ready Probe 1-Step Kit is specially formulated for ultra-sensitive RNA detection using probe-based RT-qPCR and is ideal for developing lyophilised diagnostic assays.
Optimized for Lyophilisation
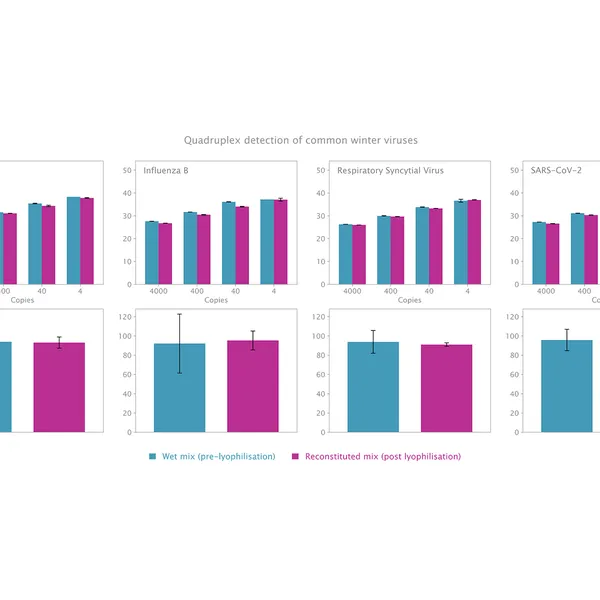
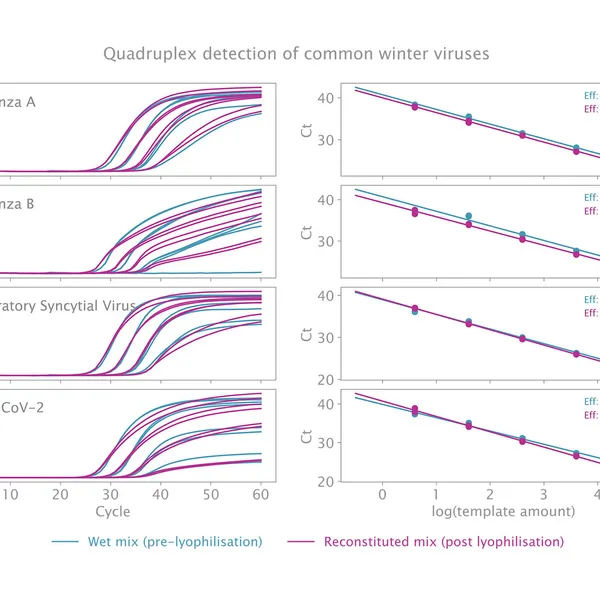
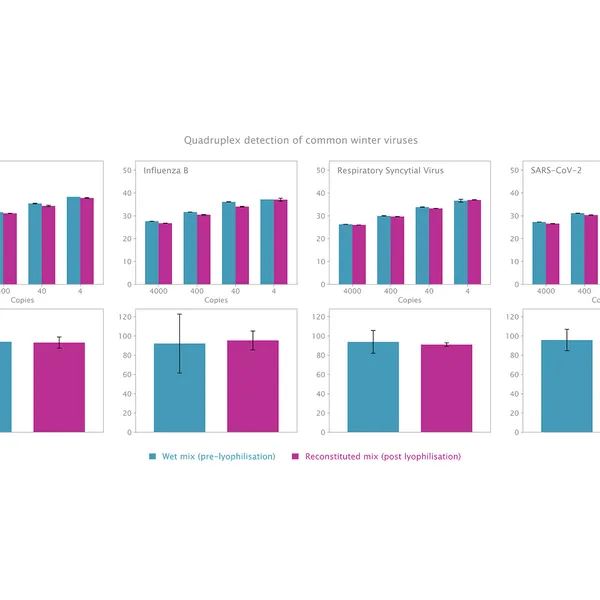
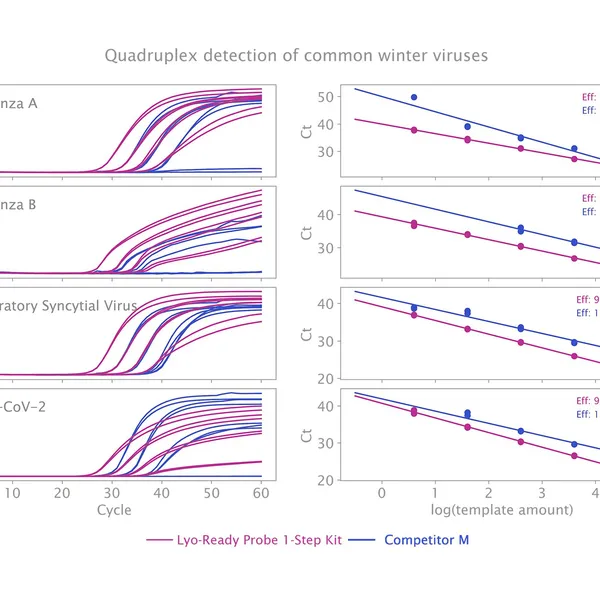
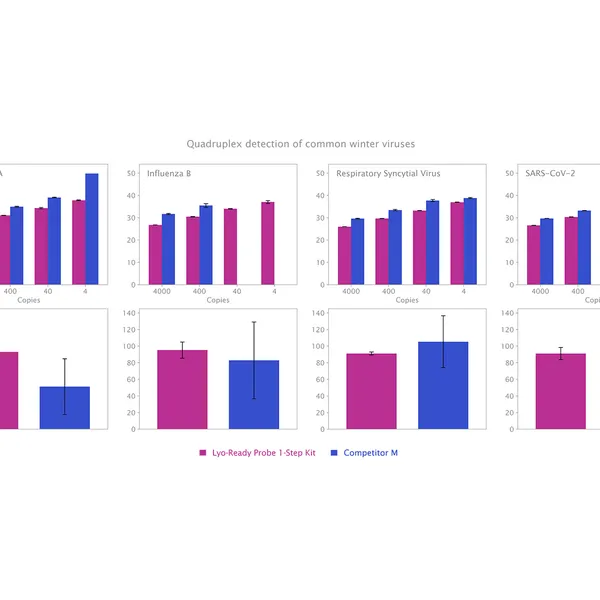
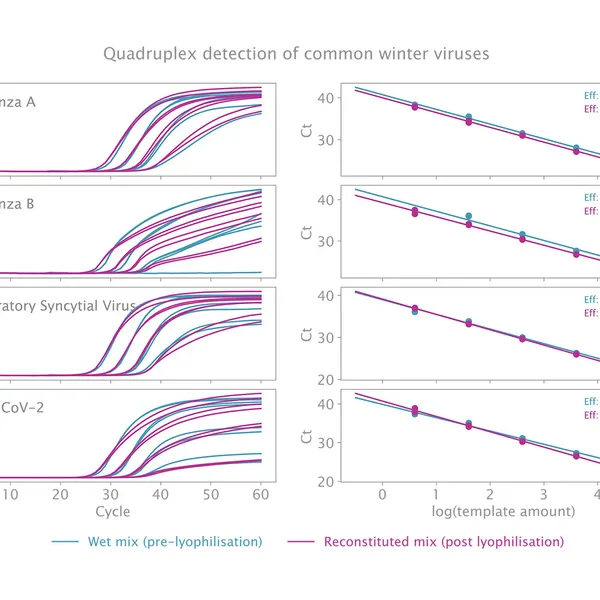
Lyophilisation offers several benefits including room temperature storage, extended shelf life, and increased flexibility in sample volumes. The kit is optimized to deliver high performance equivalent to our standard 1-step RT-qPCR kits following lyophilisation.
Robust and Reliable RT-qPCR
The Lyo-Ready Probe 1-Step Kit includes a 4x qPCR mix without glycerol, containing hot-start Taq polymerase, dNTPs, MgCl2, and a blend of excipients to ensure reliable lyophilisation without loss of activity. Intermediate antibody-mediated hot-start technology ensures specific amplification of virus-derived cDNA, with resistance to common PCR inhibitors found in clinical samples.
UltraScript® Reverse Transcriptase is provided separately in an extremely concentrated 3200x format. Specially designed to accelerate cDNA synthesis speed and efficiency with accurate representation of transcripts, this modified MMLV reverse transcriptase enzyme is thermally stable and enables fast and effective reverse transcription up to 55 °C.
SARS-CoV-2 Detection
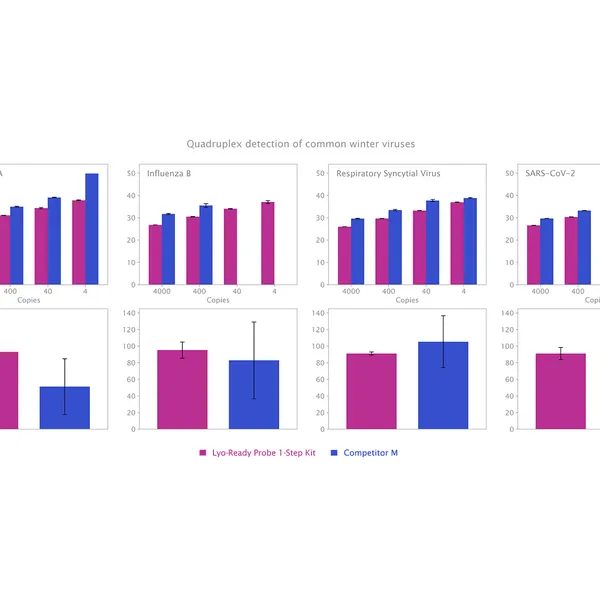
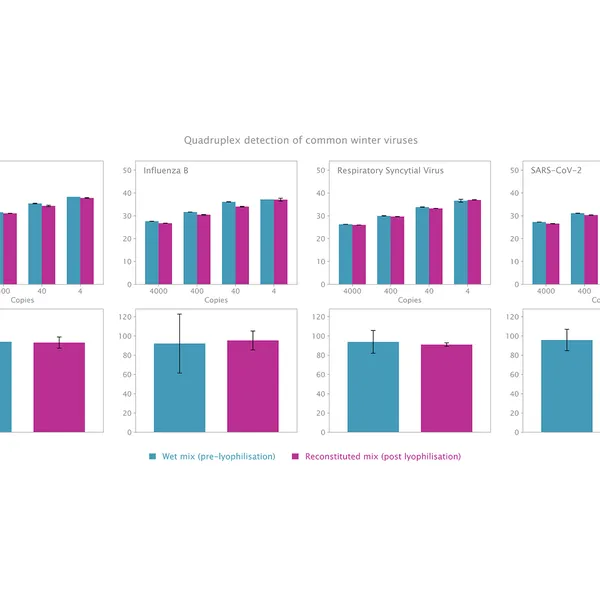
The kit has been validated for the detection of SARS-CoV-2 in singleplex (see Figure 1) and multiplex (see Figure 2) mode, providing reliable performance without loss of performance or sensitivity following lyophilisation.
Customised Diagnostic Test Development
At PCR Biosystems, we understand that every molecular diagnostic test is different and that lyophilising them can be challenging. To support your initial verification work, we offer the Pre-Lyo Probe 1-Step Evaluation Kit for testing purposes.
ISO 13485
The Lyo-Ready Probe 1-Step Kit has been manufactured under an ISO 13485 certified quality management system, and is suitable for further manufacturing as a component, reagent, or reagent pairing for molecular diagnostic assays.
Applications
- Development of lyophilised molecular diagnostic tests
- Detection of targets with extremely low copy numbers
- Compatible with TaqMan®, Scorpions®, and molecular beacon probes
Specifications
Lyo-Ready Probe 1-Step Kit
| Component | 2000 Reactions | 10 000 Reactions | 100 000 Reactions |
|---|---|---|---|
| 4x Lyo-Ready Probe Mix | 2 x 5 mL | 1 x 50 mL | 1 x 500 mL |
| 3200x UltraScript RTase (with RNase inhibitor) | 1 x 12.5 μL | 1 x 62.5 μL | 1 x 625 μL |
Pre-Lyo Probe 1-Step Evaluation Kit
| Component | 600 Reactions | ||
|---|---|---|---|
| 4x Lyo-Ready Probe Mix | 3 x 1 mL | ||
| 20x UltraScript RTase (with RNase inhibitor) | 1 x 600 µL |
Lyo-Ready Probe Mix
| Component | 600 Reactions | 2000 Reactions | 10 000 Reactions | 100 000 Reactions |
|---|---|---|---|---|
| 4x Lyo-Ready Probe Mix | 3 x 1 mL | 2 x 5 mL | 1 x 50 mL | 1 x 500 mL |
Reaction Information
| Reaction Volume | Storage Space | ||
|---|---|---|---|
| 20 μL |
Upon receipt, products should be stored between -30 and -20 °C. If stored correctly, the kit will retain full activity until the stated expiry date. |
Documents
Product Flyer
User Manuals
Frequently Asked Questions (FAQs)
What Ct value is considered unreliable?
Ct values can vary between template concentrations, reaction optimization, instrumentation, and laboratories so care should be taken when selecting a Ct cut-off value. Generally, Ct values above 35-40 will start to be considered unreliable. However, late Ct values may be observed for inefficient reactions with low template amounts. The best practice is to normalize cut-offs with relative or absolute quantification methods. It is also advisable to run and analyze melting curve or gel profiles of the products to determine product from any late amplification.
Can other components be added to the Lyo-Ready Probe 1-Step Mix prior to lyophilisation?
We have not tested any additional components for addition to the Lyo-Ready Probe 1-Step Mix apart from primers, probes, and ROX. If you wish to add other lyophilisation-compatible components, you will need to assess their activity in both the wet mix and lyophilised material, as well as evaluate their stability over time. PCR Biosystems takes no responsibility for any performance reduction resulting from added components.
Can ROX be added to the Lyo-Ready Probe 1-Step Mix and does it negatively affect the reaction?
Yes, ROX (6-carboxy-X-rhodamine), supplied separately, can be added to the kit and it will not affect lyophilisation.
ROX is used as a passive reference dye in ROX-dependent real-time PCR instruments to normalize for fluctuations in fluorescence levels which may appear due largely to optical path change between wells. Normalization of fluorescence intensity (Rn) is performed in real-time PCR software by dividing the emission intensity of the specific signal with the emission intensity of ROX.
ROX does not participate in the PCR reaction and its fluorescence levels do not correlate with template amounts in each well; thus, adding this fluorophore to the mix provides a consistent fluorescent signal during amplification.
Different real-time PCR instruments require passive reference standards with differing optimal ROX concentrations, mostly due to each system's different optical configurations (i.e., different excitation and optics types used).
Adding too much or too little ROX will result in a very noisy signal adversely affecting the reaction results. Therefore, users should:
- Identify the correct ROX concentration to optimize real-time PCR results, and
- Check the ROX settings on the software used to set up the reaction
A useful guide for choosing the appropriate system is available here.
Is it possible to change activation time for HS Taq DNA Polymerase?
We recommend using at least 2 minutes to activate the polymerase. Longer times up to 15 minutes can also be used with no detrimental effect on the enzyme.
Do I need to use an RNase inhibitor in my RT reaction?
No, 3200x UltraScript® RTase provided in the kit includes an RNase inhibitor to prevent degradation and increase sensitivity.
General troubleshooting for low product or late Ct values
If you observe unusually late Ct values, try diluting the sample RNA. By doing so, you are diluting any inhibitors that may be present at concentrations that inhibit the reaction. Additionally, try increasing the reverse transcription step to 55 °C and increasing the annealing/extension temperature. This can help alleviate difficulties caused by secondary structures within RNA samples and/or primers.
In cases where inhibition of the reaction is possible, try reducing the sample amount1 or add 0.4 – 4.4 mg/ml BSA to the reaction2.
For more specific issues, please contact technical@pcrbio.com with the following information:
- Product size
- Reaction setup
- Cycling conditions
- Screenshots of amplification traces and melt profiles
1 Scipioni et al. A SYBR Green one-step real-time RT-PCR for detection of human and bovine noroviruses and control of inhibition. Journal of Virology.5:94 (2008). doi: 1186/1743-422X-5-94
2 Plante et al. Use of bovine serum albumin to enhance detection of enteric viruses by RT-qPCR from food washing concentrates. Letters in Applied Microbiology. 52:3 (2010) doi: https://doi.org/10.1111/j.1472-765X.2010.02989.x
How should I handle the Lyo-Ready Probe 1-Step Kit?
The Lyo-Ready Probe 1-Step Kit contains 2 components: 4x Lyo-Ready Probe Mix and 3200x UltraScript® Reverse Transcriptase (RTase). 1.25 μL of 3200x UltraScript RTase should be added per mL of 4x Lyo-Ready Probe Mix used. Due to the high concentration of the RTase, the provided volume is very small, so it is advisable to spin down the tube prior to each pipetting task.
While the 2 kit components are stable and with long shelf life (see expiry dates for each component), the 4x Lyo-Ready Probe 1-Step mix (obtained by adding 3200x UltraScript® RTase to 4x Lyo-Ready Probe Mix) should be used immediately or may be stored for up to 3 days at 4 °C. Therefore, it is advisable to prepare only the amount of 4x Lyo-Ready Probe 1-Step Mix necessary and return the remaining parts separately to -30 °C to -15 °C storage.
Is it normal if the fluorescence levels of the 4x Lyo-Ready Probe Mix differ from competitors' products?
Different products can produce different fluorescence levels. However, this does not affect quantitative accuracy and Ct values will not differ between products.
What is the MgCl2 concentration in the 4x Lyo-Ready Probe Mix?
4x Lyo-Ready Probe Mix contains MgCl2 at a concentration of 18 mM. This means the final concentration in the reaction is 4.5 mM.
What type of primers can I use?
Gene-specific primers should be used in one-step reactions.
What should I do in case of problems with lyophilisation?
In the product's instructions, we provide a general lyophilisation protocol tested for 2 mL glass vials containing 500 μL of Lyo-Ready Probe 1-Step Mix. We have observed that lyophilisation is easier (i.e., shorter cycles, and cakes appear more uniform with no evidence of shrinkage) when the Lyo-Ready Probe 1-Step mixture is diluted to 1x or 2x (with oligonucleotides and water), so we suggest performing this dilution prior to running.
Depending on the lyophilised volume, the materials of the vials (i.e., glass vs plastic), and the lyophiliser used, the cycle may be optimized.
If cakes are not correctly formed after a cycle, we recommend repeating with a more conserving cycle, where primary drying is performed at -50 °C and time is lengthened to ensure correct cake formation. Secondary drying time can also be increased if necessary.
What should be considered when multiplexing with the Lyo-Ready Probe 1-Step Kit?
Primers should be designed to ensure they have similar annealing temperatures, are specific to the target, and do not form primer dimers. The reverse transcription step time can be extended to 10 minutes to ensure sufficient template is available for priming and amplification.
When multiplexing, what is the recommended concentration of each primer?
We recommend using 400-1000 nM for each primer. There is some flexibility around this recommended concentration; however, primer concentration should not be increased beyond this range as significant effects on enzyme performance may occur. Typically the probe concentration is half that of the amplification primers, but optimization and validation are necessary for each primer pair.


Read more